Research projects
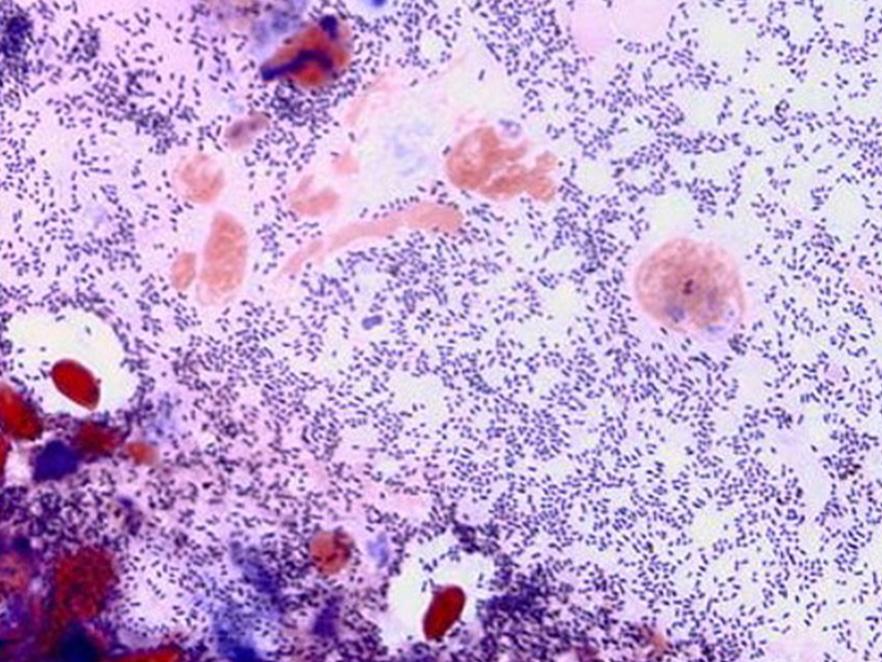
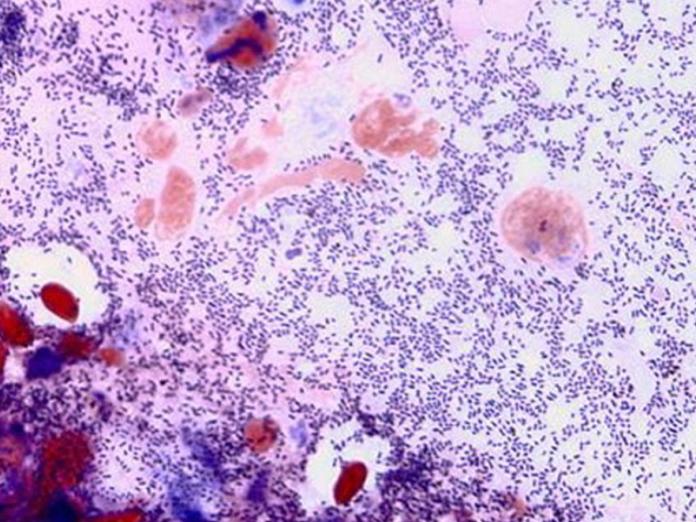
Bacterial kidney disease
Dr. John Fryer and his first graduate student (ODFW) laid much of the groundwork for Bacterial Kidney Disease (BKD). Subsequently, ODFW, in collaboration with OSU, has developed, tested and implemented screening methods for detecting BKD Renibacterium in adult salmon. BKD is a serious infection of salmon caused by the bacterium Renibacterium salmoninarum. Disease signs include characteristic off-white lesions in the kidney, that usually progress to become a systemic infection that kills the host. The disease can be transmitted from the adult female via infected eggs to the progeny and has impacted many salmon and trout populations. Culling infected eggs during hatchery spawning has been one of the most effective tools in reducing levels of the disease in juvenile salmon.
Fryer, J.L. and Sanders, J.E. 1981. Bacterial kidney disease of salmonid fish. Ann. Rev. of Microbiol. 273-298.
Banner, C.R., Long, J.J., Fryer, J.L. and Rohovec, J.S. 1986. Occurrence of salmonid fish infected with Renibacterium salmoninarum in the Pacific Ocean. J. of Fish Dis. 9:273-275.
Amandi T., and Lindsay, L. ??????? Culling of eggs from BKD positive spring Chinook females can lead to reductions of the disease in smolts and the use of medicated feed. Proc. of the 52nd Ann. Pacific NW Fish Culture Conf. P67-76.
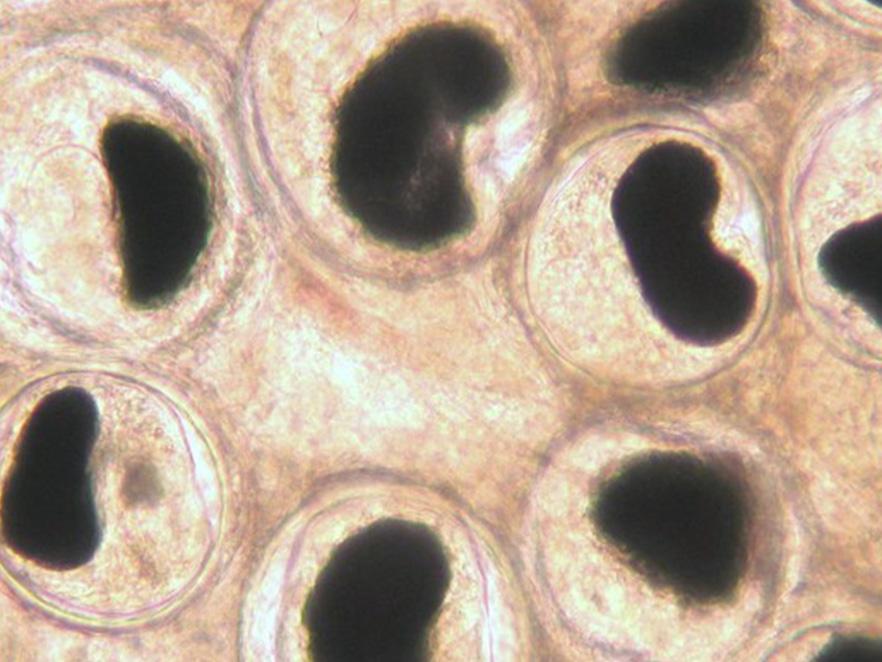
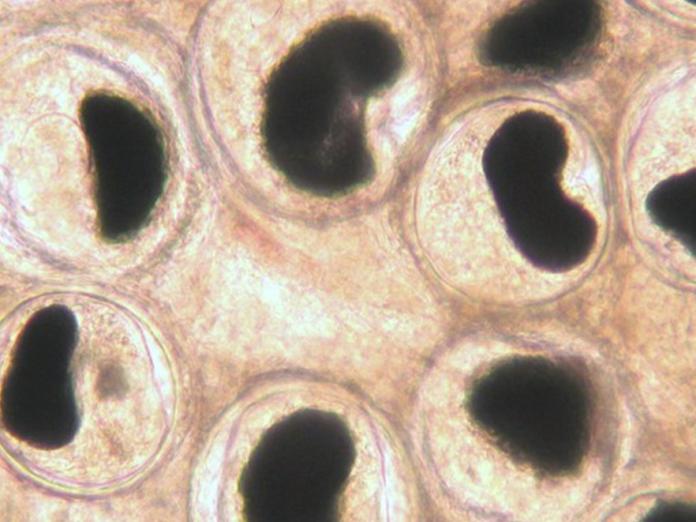
Black spot (Nanophyetus)
Researchers from the Kent and Bartholomew labs (Microbiology), as well as the Schreck lab (Fish and Wildlife) discovered that Nanophyetus infection reduced both growth and swimming ability in coho salmon. Nanophyetus salmincola is a common trematode parasite of fish in Oregon. It is known as the "salmon poisoning fluke" because it can carry the bacterium Neorickettsia helminthoeca, which can be fatal for dogs that eat uncooked infected fish. Nanophyetus has a complex life cycle that involves a snail, fish, and mammal hosts. In fish tissues it forms cysts, which appear as small black spots. High infection intensity can also increase susceptibility to bacterial infections in juvenile Chinook salmon.
Roon, S., Alexander, J., Jacobson, K. and Bartholomew, J. 2015. Effect of Nanophyetus salmincola and bacterial co-infection on mortality of juvenile chinook salmon. J. of Aquatic Animal Health, 27:209-216.
Ceratonova shasta life cycle
Dr. Jerri Bartholomew deciphered the complex C. shasta life cycle and identified the infectious agent for fishes in the mid-1990s. The complex lifecycle is now maintained in the AAHL. This myxozoan parasite (formerly Ceratomyxa shasta) causes enteronecrosis in salmon and trout. C. shasta is native to the Pacific Northwest and can cause disease outbreaks in trout hatcheries and in free-ranging Chinook and coho salmon under environmental stress. The discovery that the parasite requires a polychaete host has enabled further research on fish susceptibility, parasite genetics, and the influence of temperature and flow on infection dynamics.
Bartholomew, J., Whipple, M., Steens, D., and Fryer, J. 1997. The life cycle of Ceratomyxa shasta, a myxosporean parasite of salmonids, requires a freshwater polychaete as an alternate host. J. of Parasitology.

Chum salmon and C. shasta
ODFW's Kris Homel, in collaboration with Dr. Jerri Bartholomew (OSU Microbiology) is studying endangered chum salmon and one of the potential sources for mortality, the parasite Ceratonova shasta which has been shown to be present in the Columbia River Basin. Chum salmon, Oncorhynchus keta, populations in the Columbia River Basin were listed as threatened in 1999 under the Endangered Species Act. Despite recovery efforts, fry-to-adult survival rates remain depressed and are consistently lower than in other regions. Lab and in-river sentinel exposures of two stocks of chum salmon (Big Creek, Oregon and Washougal, Washington) show low to moderate doses of C. shasta confirming the previously unknown susceptibility of this salmon to the parasite.

Klamath River studies
Dr. Jerri Bartholomew (OSU Microbiology) collaborated on a fish health monitoring program with the Bureau of Reclamation that combined water sampling, inverterate collection and "sentinel" fish exposures to inform real-time river management decisions. The Klamath River spans the Oregon/California border and flows west into the Pacific Ocean. It is an important habitat for salmonids. Several pathogens impact the chinook and coho salmon populations. Spatial and temporal data on occurrence of fish pathogens facilitates model development, guides management decisions, and guides risk assessments.
Ray, R.A., Holt, R.A. and Bartholomew, J.L. 2013. Relationship between temperature and Ceratomyxa shasta-induced mortality in Klamath River salmonids. J. Parasitol. 9(3):520-526.
Hallett, S.L., Ray, R.A., and Hurst, C.N. 2012. Density of the waterborne parasite Ceratomyxa shasta and its biological effects on salmon. Appl. Environ. Microbiol. 8(10):3724-3731.
Chiaramonte, L., Ray, R., Corum, R., Soto, T., Hallett, S., and Bartholomew, J. 2016. Klamath river thermal refuge provides juvenile salmon reduced exposure to the parasite Ceratonova shasta. Trans. of Am. Fisheries Soc. 145:810-820.
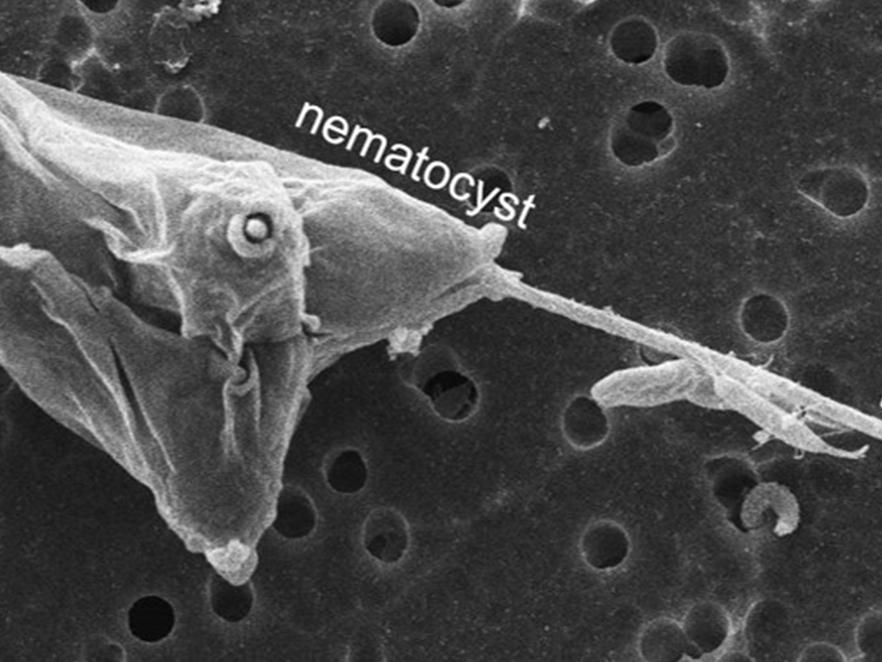
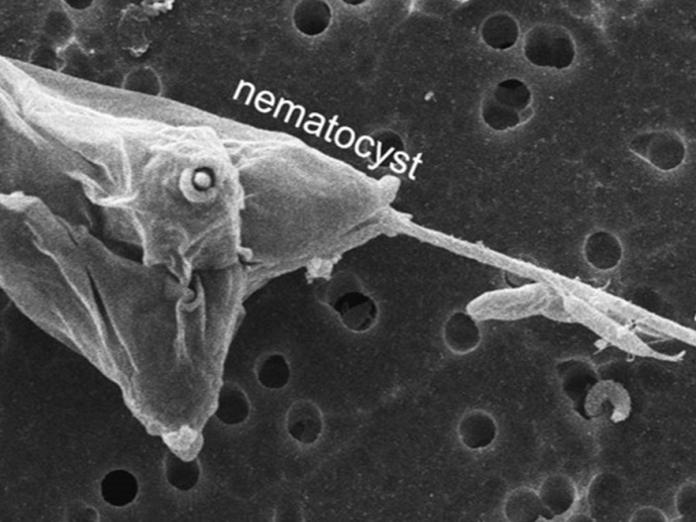
Myxozoan nematocyst discharge
Dr. Stephen Atkinson, Dr. Jerri Bartholomew and grad student Benjamin Americus (OSU Microbiology) in collaboration with the University of Haifa are researching the source of energy behind this process in other cnidarians. Myxozoans are parasitic, microscopic cnidarians. Like other cnidarians (jellyfish anemones) they have specialized stinging organs called nematocysts to attach to fish in the first step towards infection. Nematocyst discharge is driven by osmotic energy which can be negated by putting the animals in cation-rich water. With the myxozoan parasite Myxobolus cerebralis, the ability of different cations such as Na2+ and K+ to interfere with nematocyst discharge and prevent infection is being examined.
Americus, B.E. 2019. investigation of nematocyst discharge in parasitic cnidarians (myxozoa). OSU.

Whirling disease
The Bartholomew Lab established the parasite life cycle in the AAHL isolation lab, using both fish and worm hosts. Whirling Disease afflicts salmon and trout and is caused by an introduced parasite, Myxobolus cerebralis. It has had significant economic and ecological impacts on native and hatchery fish. Establishment of the complex life cycle involving an invertebrate host and 2 spore stages enabled experiments into genetic characterization of hosts, host susceptibility and the influence of temperature and water flow on infection dynamics. Water flow rate is an environmental variable affecting the establishment and propagation of M. cerebralis. Both the parasite and its invertebrate host proliferate to a greater extent in a slow flow environment. This finding is of significance for aquatic systems where the flow rate can be manipulated, and should be incorporated into risk analysis asssessments.
Hallett, S. and Bartholomew, J. 2008. Effects of water flow on the infection dynamics of Myxobolus cerebralis. Parasitology. 135:371-84.

White spot disease (Ich)
Claire Howell (OSU Bartholomew Lab, Microbiology) developed a water-sampling method that can quickly detect harmful levels of Ich in wild river systems. White spot disease is caused by the globally distributed freshwater fish parasite Ichthioptherius multifilis (Ich). Outbreaks of this disease are difficult to control, and can result in catastrophic mortality events of both wild and cultured fishes. The parasite has a direct, temperature-dependent life cycle that enables rapid multiplication when hosts are plentiful and environmental conditions are favorable. Accurate detection is central to the control of Ich infections and prevention of host mortality particularly in wild systems where chemical treatments are not feasible. A sampling method based on molecular analysis of water samples for parasite DNA was developed and tested. The small subunit ribosomal DNA (ssrDNA) of Ich isolates was collected from the Klamath River and used to develop and validate a novel qPCR assay. This method will protect vulnerable fish populations, includinng migrating adult salmon.
Howell, C.K., Atkinson, S.D, Bartholomew, J.L. and Hallett, S.L. 2019. Development and application of a qPCR assay targeting Ichthyophthirius multifiliis in environmental water samples. Dis. Aquat. Organ. 134(1):43-55.

Worms
Dr. Jerri Bartholomew (OSU Microbiology) cultured oligochaetes and polychaete worms under laboratory conditions in order to better understand the invertebrate hosts of some fish parasites which have two host life cycles. Several significant fish pathogens have life cycles that involve both fish and an alternate, invertebrate host like a snail, crustacean or worm. Long term research has involved myxozoan parasites C. shasta and M. cerebralis and has resulted in laboratory cultures of both their worm and fish hosts. Different worms require different living conditions. Oligochaetes such as Tubifex grow well in tubes of sediment. The polychaete host of C. shasta requires more elaborate flow-through or re-circulating nutrient systems.
Bjork, S.J. and Bartholomew, J.L. 2009. The effects of water velocity on the Ceratomyxa shasta infectious cycle. J. Fish Dis. 32(2):131-142.
Bartholomew, J., Atkinson, S., and Hallett, S. 2006. Invovement of Manayunkia speciosa (Annelida: Polychaeta: Sabellidae) in the life cycle of Parvicapsula minibiconis, a myxozoan parasite of Pacific salmon. J. of Parasitol.